Application and comparison information using creatinine, mandelic acid, hippuric acid, and methylhippuric acid
Comparison of stationary phases using isomers of methylhippuric acid
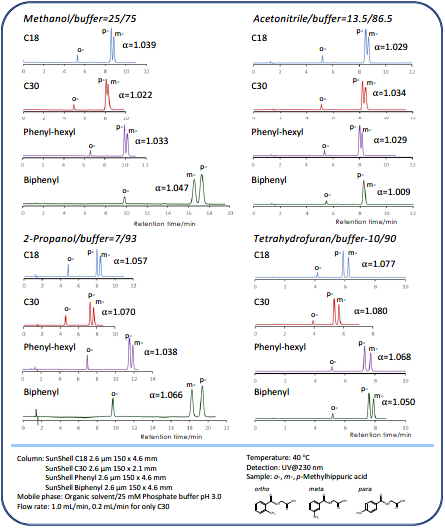
The separation of ortho, meta, and para isomers of methyl hippuric acid was compared. The stationary phase was C18, C30, Phenyl-hexyl, and Biphenyl, and the organic solvent in the mobile phase was methanol, acetonitrile, 2-propanol, and tetrahydrofuran. The separation of meta-methyl hippuric acid and para -methyl hippuric changed depending on the organic solvent, and the separation was improved in the order of acetonitrile, methanol, 2-propanol, and tetrahydrofuran. Biphenyl showed particularly high retention when alcohol was used as the organic solvent in the mobile phase and the elution order and para were reversed from that of other stationary phases. It is considered that this is due to the high hydrogen-bonding capacity obtained on comparison of separation of standard samples. When acetonitrile is used, the π-π interaction between the solute and the stationary phase is weakened by the triple bond of CN in acetonitrile, so it is considered that the characteristics of Biphenyl cannot be fully exhibited. When tetrahydrofuran is used, tetrahydrofuran enters the stationary phase, and a mixture of biphenyl group and tetrahydrofuran works as a stationary phase, so it is considered that the separation behavior was different from that when alcohol was used.
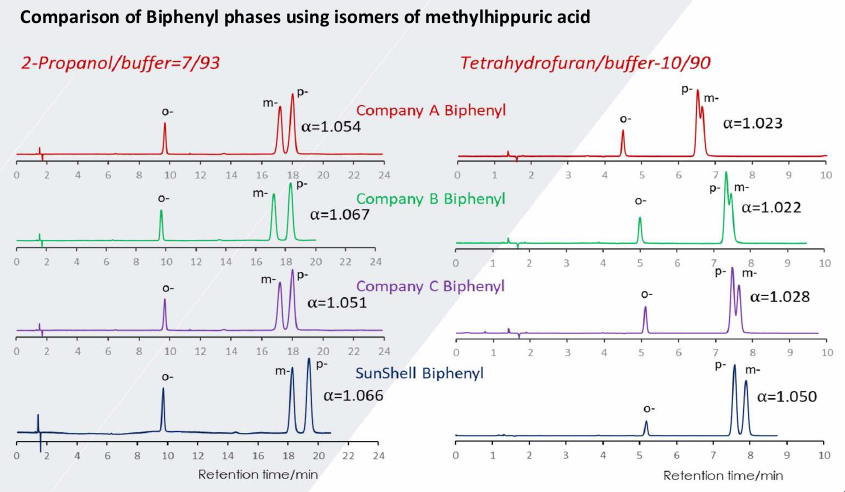
Comparison of Biphenyl phases using isomers of methylhippuric acid
Comparison of stationary phases using creatinine, mandelic acid, hippuric acid, and methylhippuric acid.
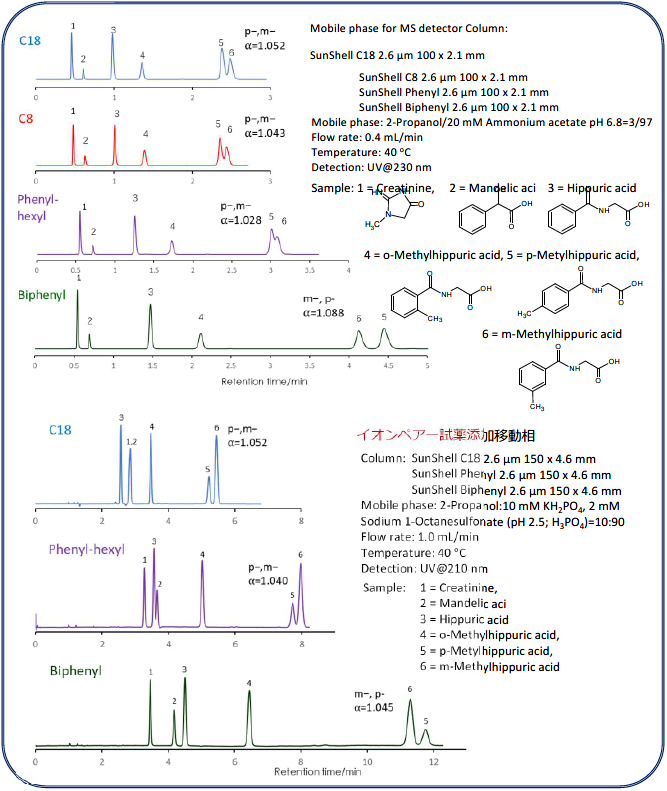
Phosphate buffer was used as the mobile phase for separation of methylhippuric acid isomers on the previous page, but in this comparison of the separation including creatinine, the ammonium acetate buffer mobile phase that can be applied to LC/MS and the mobile phase in which an ion pair reagent were added to a phosphate buffer solution was used. 2-propanol was used as the organic solvent. Under both mobile phase conditions, Biphenyl showed the highest retention and the best separation. A characteristic separation of Biphenyl is that the elution order of the isomers meta-methylhippuric acid and para-methylhippuric acid is reversed to that of C18,C8 and Phenyl-hexyl.
