212-204-0075
info@pyvot.tech
Electromembrane Extraction (EME)
How does it work?
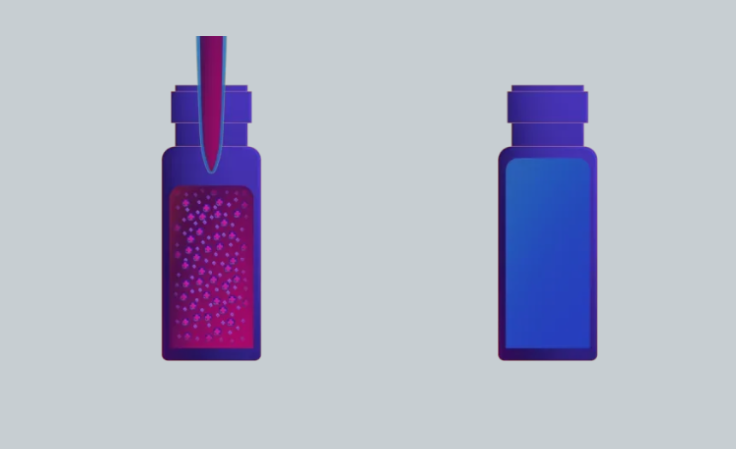
Two separate vials holding the sample solution and the acceptor solution.
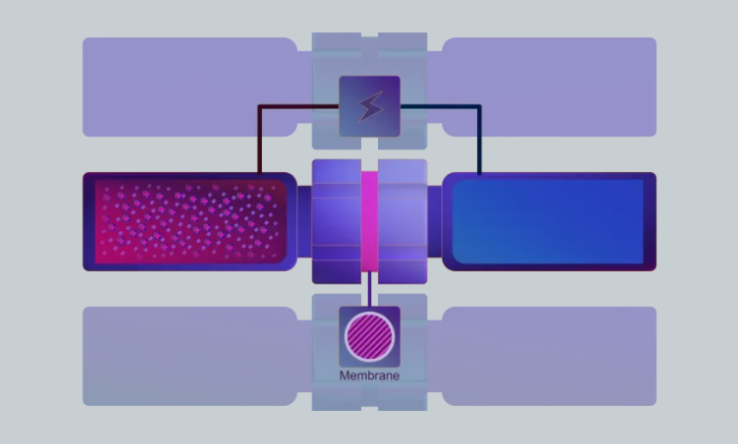
The vials are separated by an organic solvent immobilized in the pores of a polymeric membrane, called the supported liquid membrane (SLM).
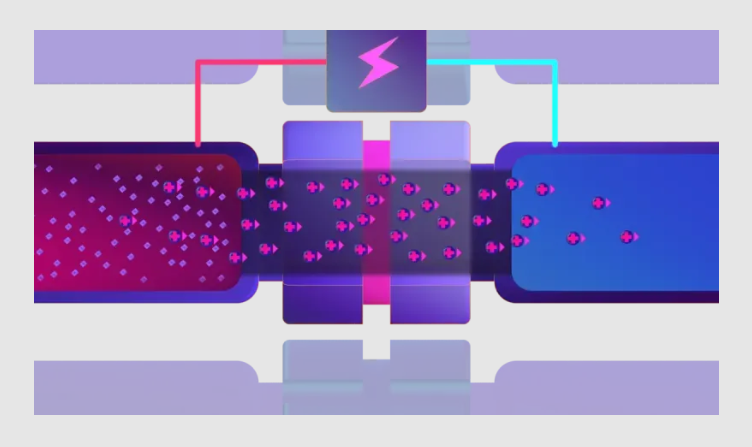
3. Apply electric field
By applying an electric field across the membrane, charged analytes are transported by electrokinetic migration from the sample solution into the acceptor solution, while non-ionic analytes and ions of opposite charge are withheld in the sample solution.

You now have pure sample extract. Under optimized conditions, EME can provide very clean extracts with reduced matrix effects i.e. a complete discrimination of proteins and phospholipids, reducing downtime of your MS instruments. The extracts can be injected directly on LC-MS instruments using very short LC methods before MS.
EME has the potential to become responsible for the paradigm shift because the technology provides:
EME has the potential to become responsible for the paradigm shift because the technology provides:
Why use Electromembrane Extraction (EME)?
EME provides rapid extractions, efficient sample clean-up and high selectivity for ionizable compounds from blood, other body fluids and environmental samples. The technique will be perfect for automation in laboratories.
The main advantages of EME are:
Selectivity and Specificity
One of the key advantages of electromembrane extraction is its exceptional selectivity and specificity. Unlike solid-phase extraction (SPE) and liquid-liquid extraction (LLE), which rely on interactions between analytes and stationary phases or solvent phases, respectively, EME leverages both electromigration and membrane permeation mechanisms. This unique combination allows EME to selectively extract target analytes based on their physicochemical properties, minimizing interference from matrix components and enhancing analytical sensitivity.
Minimal Solvent Usage and Environmental Impact
EME offers a significant reduction in solvent consumption compared to traditional methods such as SPE and LLE. In SPE, large volumes of organic solvents are often required to elute analytes from the sorbent material, leading to considerable solvent waste and environmental impact. Similarly, LLE involves the use of two immiscible liquid phases, further exacerbating solvent usage. In contrast, EME operates with minimal solvent volumes, typically in the microliter range, thereby reducing solvent waste and aligning with the principles of green analytical chemistry.
Rapid Extraction Kinetics
Time usage is of the essence in analytical chemistry and EME excels in this regard with its rapid extraction kinetics. While SPE and LLE may require prolonged extraction times to achieve adequate analyte recovery, EME’s accelerated extraction process not only increases laboratory productivity but also minimizes the risk of analyte degradation, ensuring the integrity of analytical results.
Compatibility with Small Sample Volumes
EME offers compatibility with small sample volumes, making it ideal for applications where sample availability is limited. In contrast, traditional methods such as SPE and LLE may necessitate larger sample volumes to achieve sufficient analyte recovery. By requiring only a small volume of sample, EME reduces sample consumption and facilitates the analysis of precious or scarce samples, particularly in fields such as clinical diagnostics and forensic analysis.
Versatility and Adaptability
Another advantage of EME lies in its versatility and adaptability across diverse analytical applications. Whether analyzing pharmaceuticals, environmental samples, biological fluids, or complex food matrices, EME can be tailored to suit a wide range of sample types and analyte classes. Researchers have demonstrated the efficacy of EME in extracting various analytes, including drugs, metabolites, pesticides, and environmental contaminants, underscoring its versatility in analytical chemistry.
In conclusion, electromembrane extraction represents a paradigm shift in sample preparation, offering compelling advantages over traditional methods such as solid-phase extraction and liquid-liquid extraction. With its exceptional selectivity, minimal solvent usage, rapid extraction kinetics, compatibility with small sample volumes, and versatility, EME is poised to revolutionize analytical chemistry. As researchers continue to innovate and refine this technique, the adoption of EME is expected to accelerate, driving advancements in analytical science and enabling new discoveries across diverse fields.
EME has the potential to become responsible for the paradigm shift because the technology provides:
- Excellent reproducibility
- Pure extracts
- Green sample preparation method
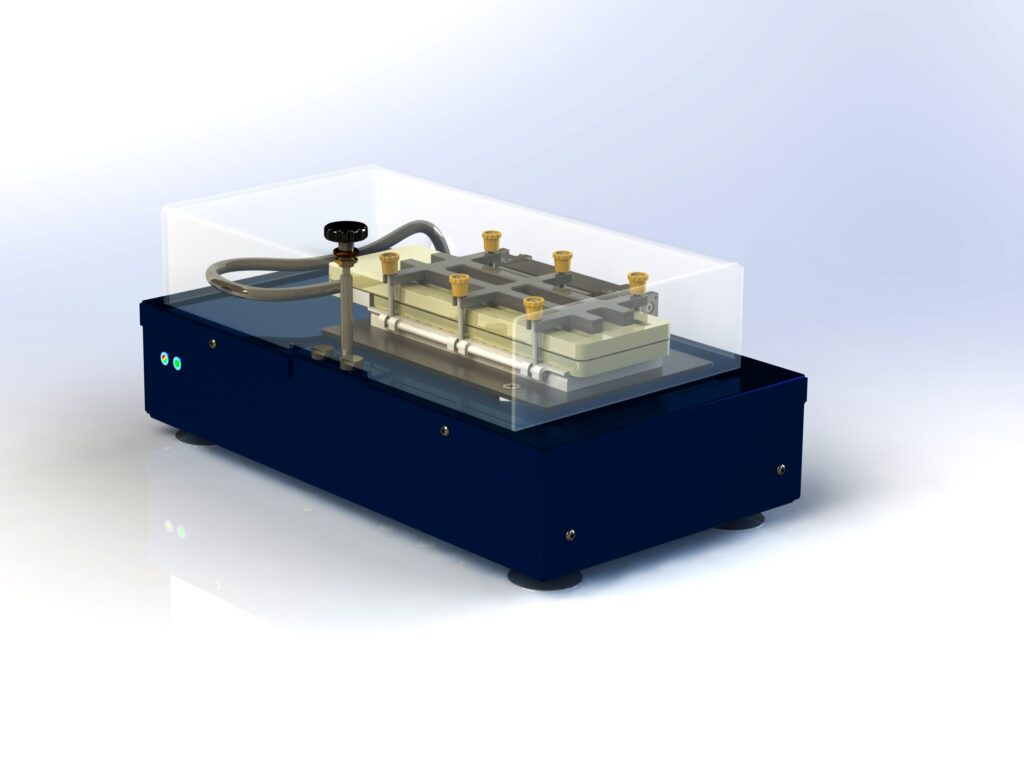
Features:
Standardizing EME
We’ve streamlined the EME process, making it ideal for R&D and application development.
Complete Extraction Kit
To simplify ETN-12 EME usage, we provide a complete extraction kit that includes vials, membranes, unions, and a vial holder.
Environmentally Friendly
Our “green” sample preparation method uses low power and minimal organic solvents.
Fast and Selective
EME enables quick and selective extraction of ionizable compounds from complex matrices.
Multi-Sample Capable
You can extract 12 samples simultaneously. Future devices will support the use of 96-well plates.
Single-Step Sample Preparation
ETN-12 EME simplifies your sample preparation to a single step, delivering pure extracts ready for analysis.
Find out here if EME is suitable for your analytes
