212-204-0075
info@pyvot.tech
Electromembrane Extraction (EME) Method Development
Method Development and Procedure
Developing an effective Electromembrane Extraction (EME) method involves fine-tuning several key parameters to ensure optimal performance. These include:
- Supported Liquid Membrane (SLM) solvent
- Sample and acceptor solution composition and volume
- Shaking rate
- Extraction voltage
- Extraction time
While specific applications may require tailored setups, generic EME methods are readily available to help you get started quickly.
To simplify method selection, how-to-eme.com provides a searchable database. Just enter a compound name to access recommended generic EME protocols along with technical documentation and additional resources.
Example: Hydrophilic vs. Hydrophobic Compound Extraction
To demonstrate EME in action, consider the extraction of:
- Hydrophilic metabolites such as choline, L-tryptophan, pyridoxine, and tyramine
- Hydrophobic drugs including desmethyl-doxepin (DM-doxepin), doxepin, trimipramine, and amitriptyline
Due to the differences in hydrophobicity, two separate generic methods are recommended:
- Method B1 – optimized for hydrophobic basic drugs
- Method B3 – designed for hydrophilic basic metabolites
A third option, Method B2, is available for compounds with moderate hydrophobicity.
These generic base methods (“B” for base-type compounds) allow for rapid and consistent method development. A summary of B1 and B3 parameters is available in Table 1, and a detailed explanation of each parameter’s role is provided in the sections below.
| Parameter | Method B1 | Method B3 |
|---|---|---|
| Applicable log P range | 2.5 ≤ log P ≤ 6.0 | -2.0 ≤ log P ≤ 2.0 |
| SLM composition | 2-nitrophenyl octyl ether (NPOE) | 6-methylcoumarin thymol 2-undecanone di(2-ethylhexyl) phosphate (17:33:49:1 weight ratio) |
| Sample | 175 µL plasma + 175 µL 200 mM formic acid | 175 µL plasma + 175 µL 900 mM formic acid |
| Acceptor solution | 350 µL 100 mM formic acid | 350 µL 450 mM formic acid |
| Voltage | 100 V | 40 V |
| Time | 30 min | 30 min |
| Agitation rate | 1000 RPM | 1000 RPM |
EME Extraction Settings
- Voltage: 10–150 V, tuned for the specific analyte.
- Shaking Speed: 1000 RPM is applied to drive the extraction.
- Most analytes migrate into the acceptor phase during the first half of the time to equilibrium, which depends on analyte properties, matrix, and sample volume.
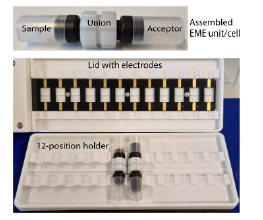
Instrumentation
Extractions were performed using the ETN-12 EME system from Extraction Technologies Norway AS, which allows up to 12 samples to be processed in parallel, facilitating high-throughput workflows.
UHPLC-MS/MS Analysis
After extraction, samples are analyzed via UHPLC-MS/MS using a C18 column (50 mm x 2.1 mm) with a fast 5-minute run time, making the process efficient and mass spectrometry-ready.
Recovery, Repeatability, and Matrix Effects in Electromembrane Extraction (EME)
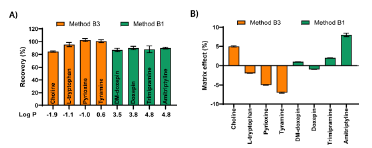
High Recovery and Excellent RepeatabilityTo evaluate the efficiency of the EME method, recovery (R) was calculated using the formula:R = (A / B) × 100%
- A = peak area of the extracted analyte from the acceptor solution
- B = peak area obtained after extracting a blank plasma sample and spiking it to 100 ng/mL

This approach compensates for matrix effects—interferences from other components in the biological sample.
Repeatability, or method precision, was assessed using the relative standard deviation (RSD) across four replicates:
RSD = (Standard deviation / Mean peak area) × 100%
Low RSD values (1.2–6.0%) indicate excellent method consistency.
Matrix Effect (ME) Evaluation
Matrix effects were calculated using the equation:
ME = [100% – (B / C) × 100%]
- C = peak area from a pure standard solution directly injected
This metric shows how much signal suppression or enhancement is caused by co-extracted substances from the matrix. Results showed minimal matrix effects between –7% and +8%, demonstrating effective matrix cleanup.
Key Results
- Recoveries: 84–102% across two EME methods
- Repeatability (RSD): 1.2–6.0%
- Matrix Effects: –7% to +8%
- Hydrophilic analytes were eluted just after the LC system’s dead volume, supporting fast separation with minimal interference.
Cleaner Biofluid Samples with EME: Reduce Matrix Effects and Protect Your LC-MS System
Electromembrane Extraction (EME) offers a powerful solution for cleaning up complex biological samples such as urine and plasma—making it an ideal sample preparation technique for LC-MS workflows.
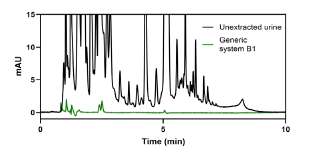
In Figure 4, the chromatogram of an untreated urine sample shows a dense mix of interfering compounds. After applying EME using the generic Method B1, the resulting extract is noticeably cleaner, simplifying analysis and improving signal quality.
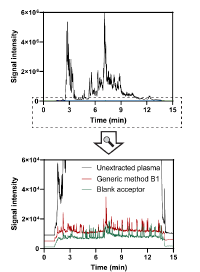
Figure 5 demonstrates EME’s effectiveness in removing phospholipids from human plasma—a major source of matrix effects in LC-MS. Phospholipids tend to accumulate on analytical columns, leading to reduced sensitivity, ion suppression, and shorter column life. EME efficiently eliminates these problematic components, delivering cleaner samples and more reliable results.
Whether you’re working with urine, plasma, or other challenging biofluids, EME enhances sample purity, protects your analytical equipment, and improves overall data quality.
Summary and Conclusion
Electromembrane Extraction (EME) is an innovative and environmentally friendly sample preparation technique that enables efficient one-step isolation of both acidic and basic analytes from complex samples, including biological fluids. While EME is widely used for small molecule analysis, it has also shown promising results for peptide extraction. With expanding potential in areas like bioanalysis and environmental testing, EME offers a versatile and sustainable alternative to traditional extraction methods.
