212-204-0075
info@pyvot.tech
Evaluation of Retention Behavior and Stability of Novel Trifunctional Biphenyl Phase
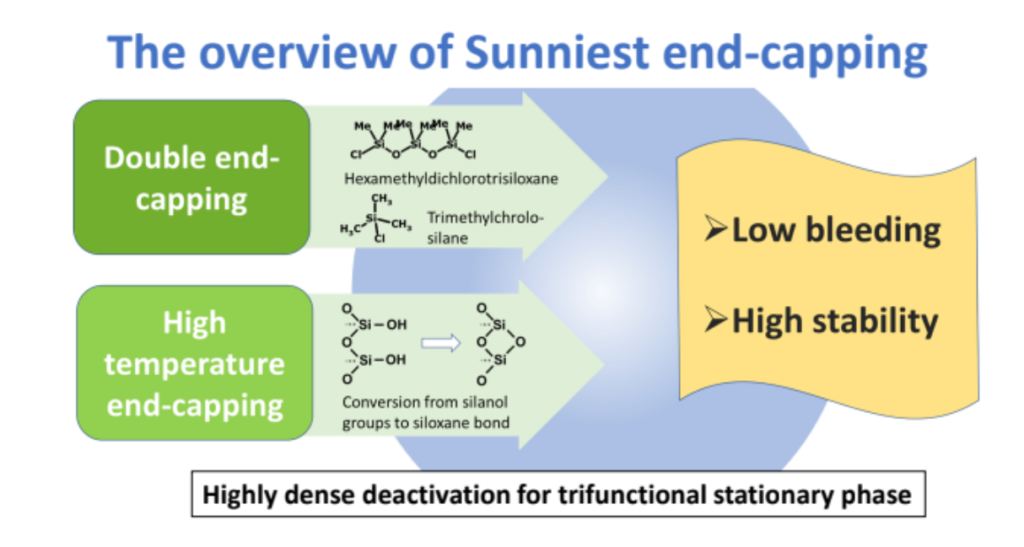
Various manufacturers of HPLC columns currently offer biphenyl columns with mono-functional biphenyl phases. However, in this study, a tri-functional biphenyl stationary phase was synthesized on a core shell silica, and double end-capping was performed at a high reaction temperature. The tri-functional biphenyl stationary phase was compared with the mono-functional biphenyl stationary phase for not only measuring hydrogen bond capacity, hydrophobicity, and steric selectivity but also assessing the peak shape of a metal chelating compound and a basic compound. Additionally, the stability of each biphenyl stationary phase was evaluated under both acidic and basic pH conditions.
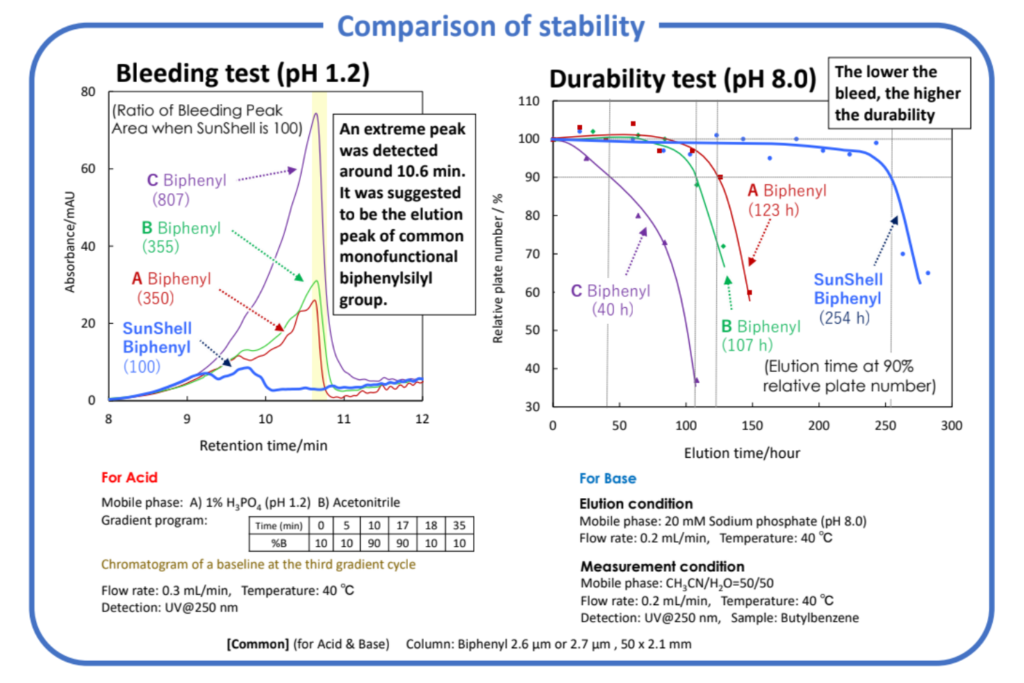
Although phenyl stationary phases exhibit higher hydrogen bond capacity than alkyl stationary phases, biphenyl stationary phases demonstrated the highest hydrogen bond capacity. This high hydrogen bond capacity led to unique separation selectivity when separating o-, m-, p-methylhippuric acid and vanillin. The proposed tri-functional biphenyl stationary phase demonstrated the most stability under both acidic and basic pH conditions.
| Column | Particle size (μm) | Particle type | Pore size (nm) | Specific surface area (m2/g) | Carbon Loading (%) | Satationary phase | End-capping | pH range |
|---|---|---|---|---|---|---|---|---|
| Company A Biphenyl | 2.6 | SPP | 10 | 200 (eff.) | 11 (eff.) | Biphenyl | TMS | 1.5 - 8.5 |
| Company B Biphenyl | 2.7 | SPP | 9 | 135 | 7 | Biphenyldimethylsilane | Yes | 1.5 - 8.0 |
| Company C Biphenyl | 2.7 | SPP | 9 | 130 | 7 | Biphenyldimethylsilane | Yes | 1.5 - 8.0 |
| SunShell Biphenyl (As S Biphenyl) | 2.6 | SPP | 9 | 150 | 5 | Trifunctional Biphenyl | Sunniest end-capping | 1.5 - 9 |
| SunShell Phenyl | 2.6 | SPP | 9 | 150 | 5 | Trifunctional Phenyl-hexyl | Sunniest end-capping | 1.5 - 9 |
| SunShell C18 | 2.6 | SPP | 9 | 150 | 7 | Trifunctional C18 | Sunniest end-capping | 1.5 - 10 |
Experiment
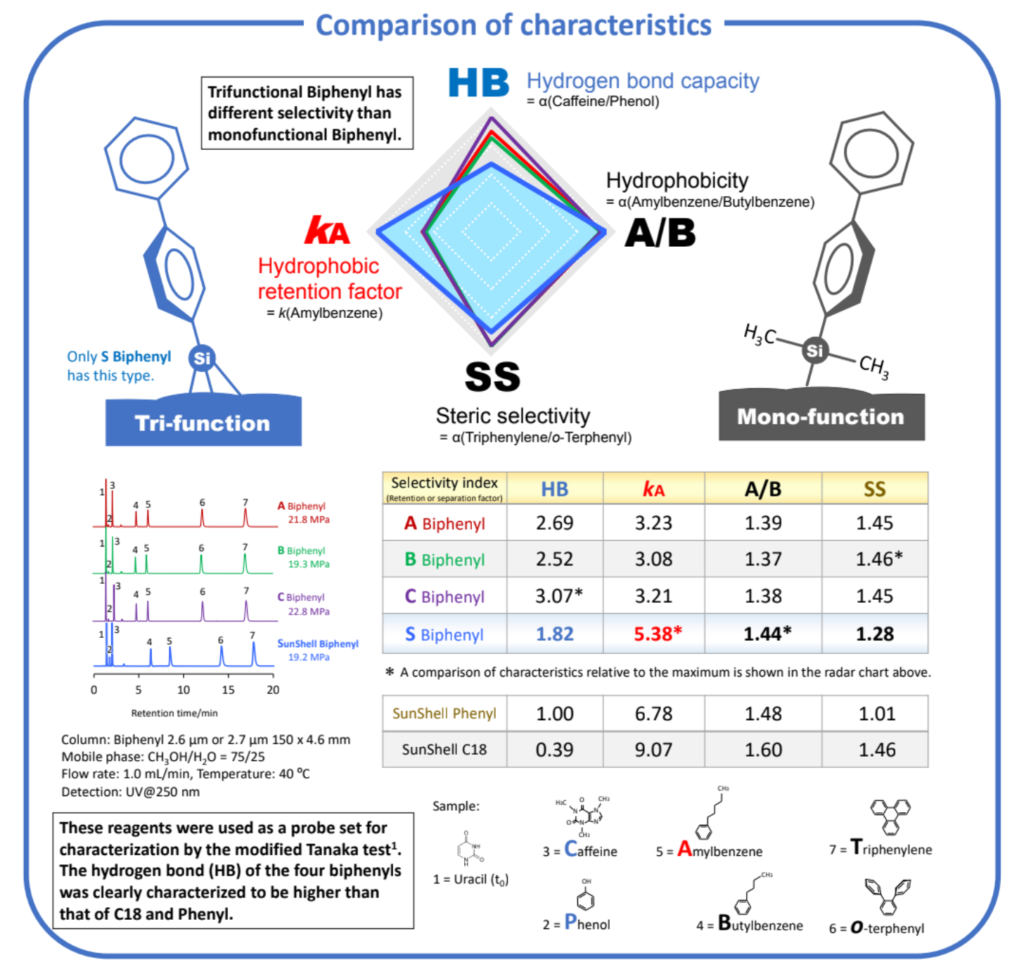
Tri-functional biphenyl stationary phase was compared with mono-functional
biphenyl stationary phases and evaluated by multiple viewpoints to measure
not only hydrogen bond capacity, hydrophobicity and steric selectivity but also
a peak shape of a metal chelating compound, an acid and a basic compound.
Furthermore, the stability of each biphenyl stationary phase was evaluated under
both acidic and basic pH conditions. Similar compounds such as isomers were
also separated to confirm the feature of biphenyl selectivity.


Conclusion:
Although the phenyl stationary phase showed higher hydrogen bond capacity (HB)
than alkyl stationary phases, the biphenyl stationary phase showed the highest
hydrogen bond capacity. Biphenyl led to unique separation selectivity when
separating methylhippuric acids, vanillins and sulfonamides. All biphenyl
stationary phases exhibited similar retention behavior for methylhippuric acids
although the HB value as hydrogen bond capacity was different. The proposed
trifunctional biphenyl stationary phase showed the most stable under both
acidic and basic pH conditions. Biphenyl exhibited unique selectivity to unable
to be explained by hydrogen bond capacity alone. We propose to call it CH-π
selectivity, which refers to the retention caused by the CH-π interaction
brought about by the biphenyl stationary phase and the effect of partial inhibition
by intramolecular interaction.
