212-204-0075
info@pyvot.tech
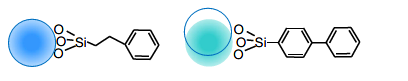
Interaction of L11 stationary phase (Phenyl, Biphenyl)
| Types of interactions | Origin of force | Feature | |||||||
| van der Waals force |
|
| Attenuates at -6th power of distance and makes an important contribution only at short distances. Dispersion is dominant in nonpolar molecules. | ||||||
| Charge transfer interaction | Electron donor-Electron acceptor | Coloring by charge transfer absorption | |||||||
| Hydrogen bond | Functional groups such as OH and NH | Strong specificity and directivity | |||||||
| Hydrophobic interaction | Non-polar groups in polar solvents | Entropy domination |
As shown in the figure above, benzene has the highest electron density in the center, and the phenyl and biphenyl
groups of the L11 stationary phase have a dipole-dipole interaction, which is the orientation force of the van der
Waals force. It then exhibits characteristic selectivity by the π-π and CH-π interactions that are treated as part of
them.
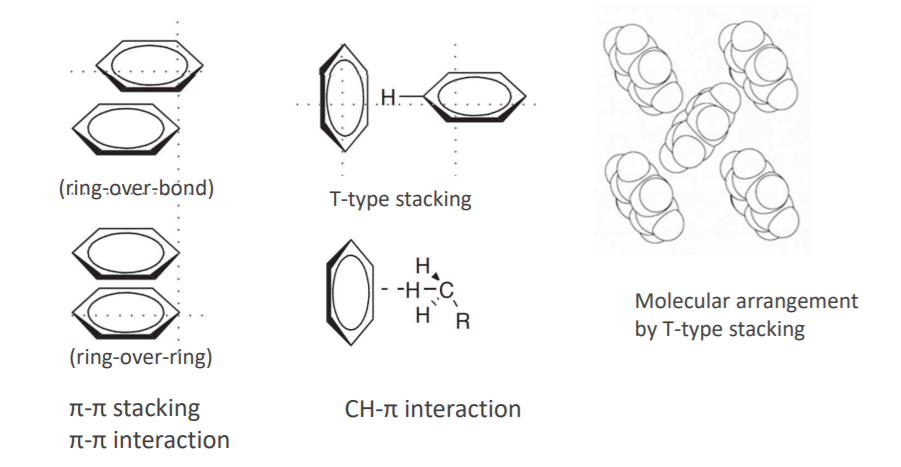
★π-π stacking and T-type stacking are known as benzene arrangement states as π-π interactions. It is presumed that
T-type stacking is close to the CH-π interaction, which is the interaction between the central part of benzene and
hydrogen of CH.
