212-204-0075
info@pyvot.tech
CERi L-Column 3
Features:
Characteristic of L-column3
| Particle | PCS silica |
| Particle size | 2μm, 3μm, 5μm |
| Pore size | 12 nm |
| Surface area | 340 m2/ g |
| Carbon contents | 17% (C18) |
| Bonded phase | C18, C8 |
| End-capping | Perfectly stable end-capping |
| USP category | L1, L7 |
| Usable pH range | pH 1 – pH 12 |
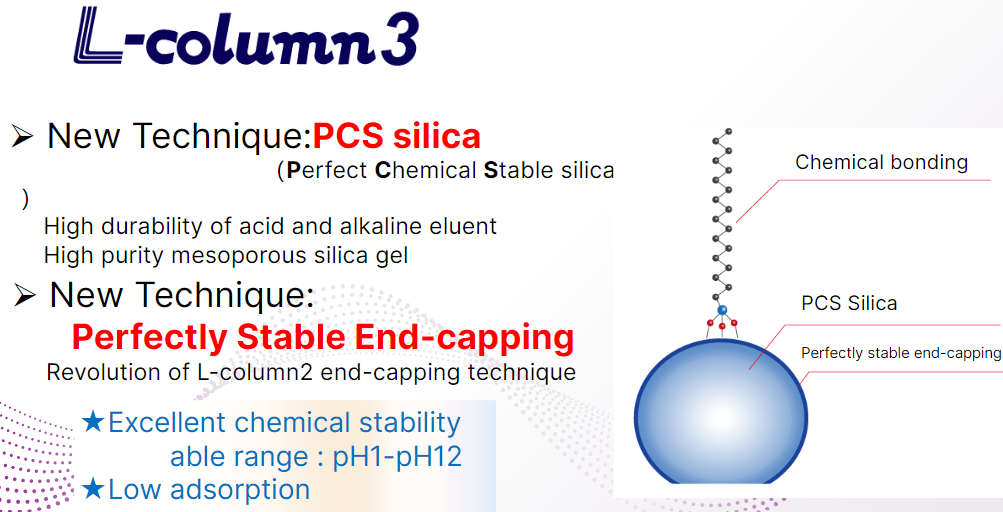
L-column3 is made entirely of PCS silica, which allows the use of common particle sizes and chemical bonding groups.
Pore size and specific surface area are comparable to those of the L-column series.
L-column3 is Recommended Below
- Start new project
- Use alkaline eluent
- Develop an advanced analytical method
- Retain basic compounds
- Change separation patterns
- Develop detection intensity of mass spectrometry
Performance:
Durability Test For L-column3 Under Alkaline Condition
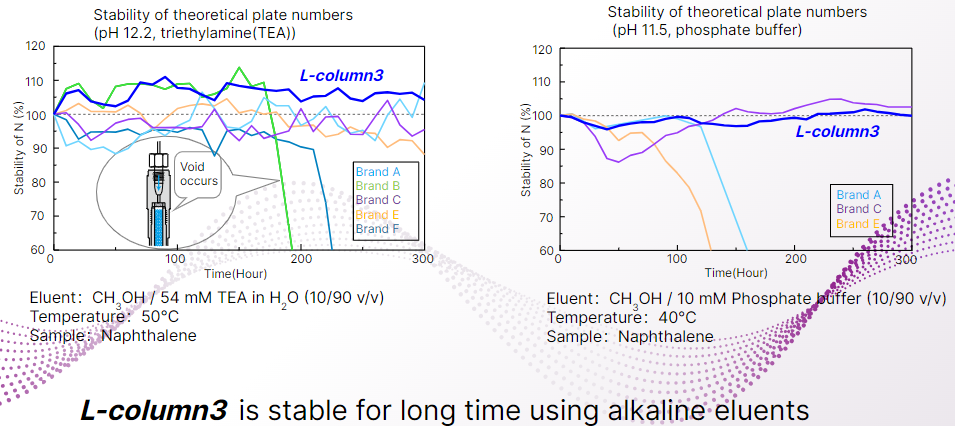
Here is a graph of a flow-through degradation test using L-column 3 and an alkali-resistant column from other companies.
In the graph on the left, 90% of the eluent was triethylamine solution at pH 12.2, which was passed through the column at a column temperature of 50°C.
In this test, the number of theoretical plates of naphthalene is measured every 10 hours to monitor column degradation due to alkaline eluents.
L-column 3 in the blue line showed extremely high alkaline resistance, as the number of theoretical plates of naphthalene remained stable after more than 300 hours.
In addition, the graph on the right shows the degradation test of phosphate buffer solution at pH 11.5 as well as triethylamine using a new column.
The results of the phosphate buffer degradation test indicate that L-column3 is stable for more than 300 hours even when the eluent composition is changed.
Comparison of End-capping Performance
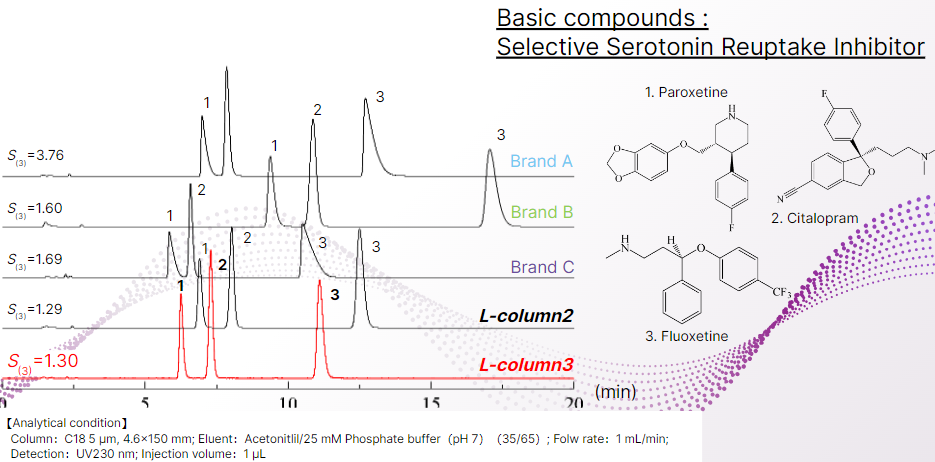
For basic substances, the presence of residual silanol groups in the column filler acts as an adsorption point, causing retention time delays and tailing.
The symmetry coefficient of fluoxetine, the third eluted substance in the chromatogram, was lower than that of other brands and almost the same value as that of L-column2.
This indicates that L-column 3 has the performance of low adsorption, which is the most important feature of advanced end-capping, and that it can also be used at high pH.
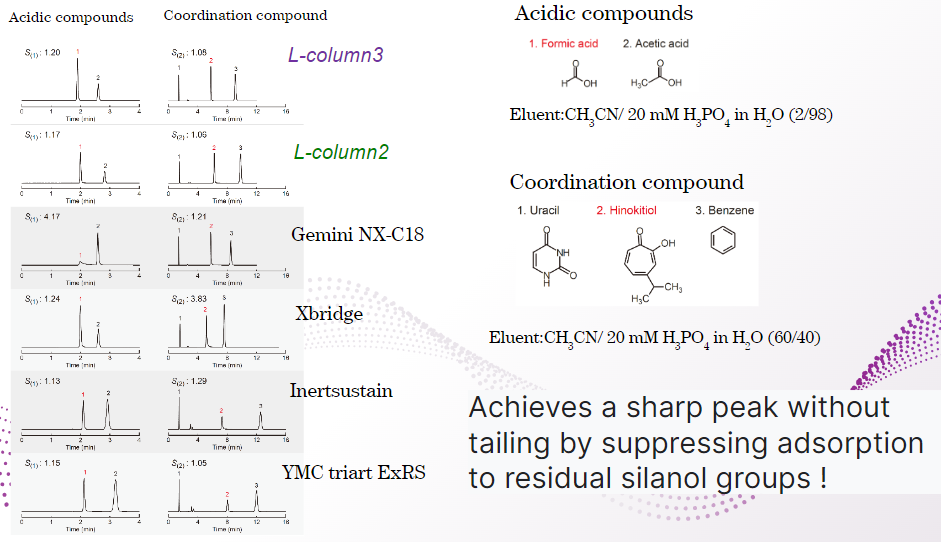
L-column 3 showed the same sharp peaks as L-column 2.
This indicates that adsorption by residual silanol groups and metal impurities is suppressed by end-capping.
Low Pressure and High Numbers of Theoretical Plate
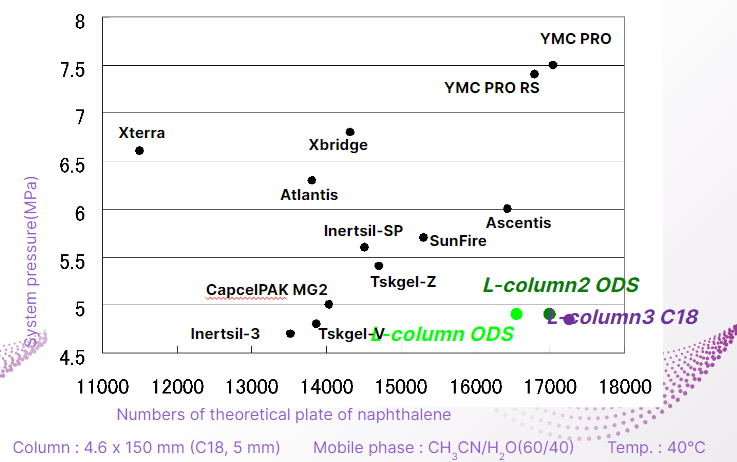
The higher the number of theoretical plates, the sharper the peak.
L-column 3 maintains a high number of theoretical plates and can be analyzed with low system pressure.
Ideal ODS column
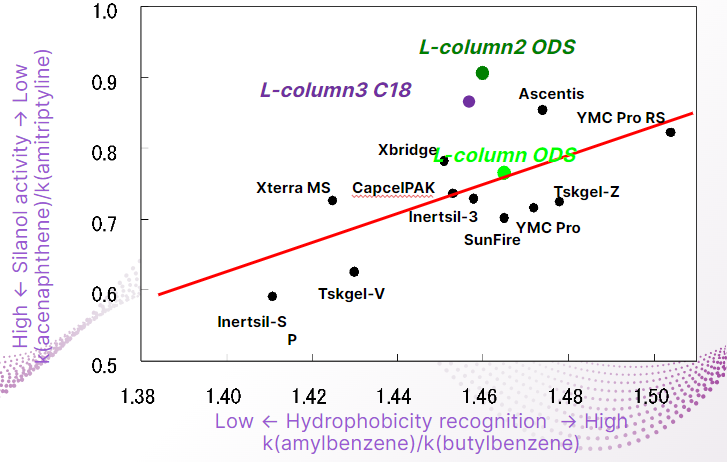
The larger the vertical axis, the lower the silanol activity. In other words, it is a column with less basic adsorption.
The larger the horizontal axis, the greater the resolution due to hydrophobic interactions.
As a feature of L-column2, the hydrophobicity recognition ability is similar to that of other companies, but the silanol activity is the lowest.
L-column3 shows values close to those of L-column2 and maintains low adsorption.
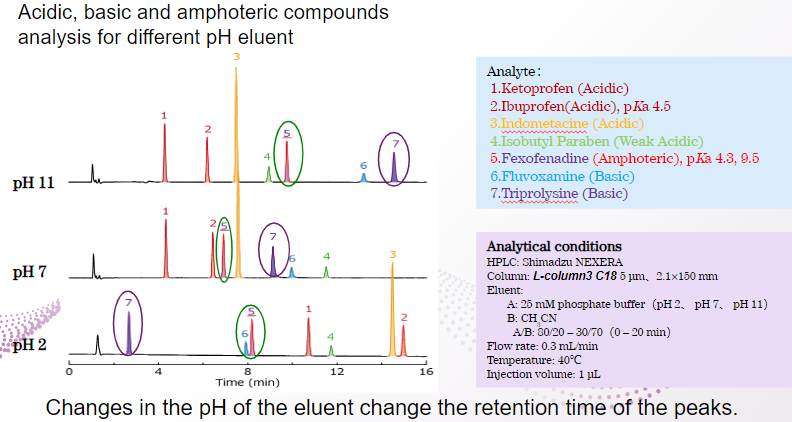
Feature of L-column3
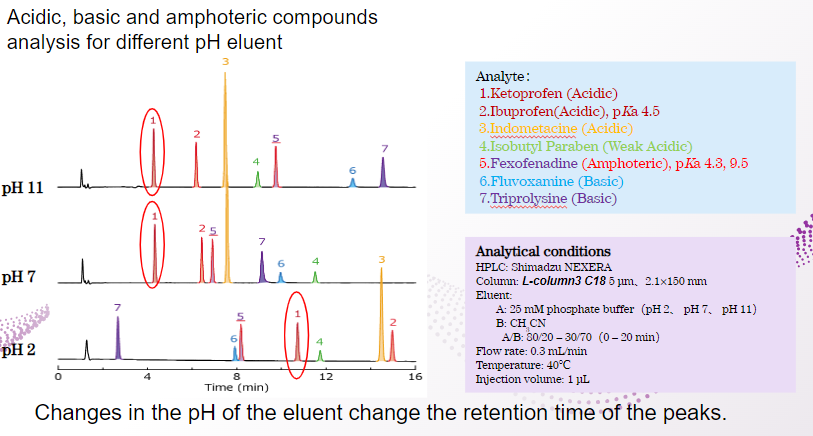
Acidic substances can be retained under acidic conditions, but not so much under neutral or alkaline conditions.
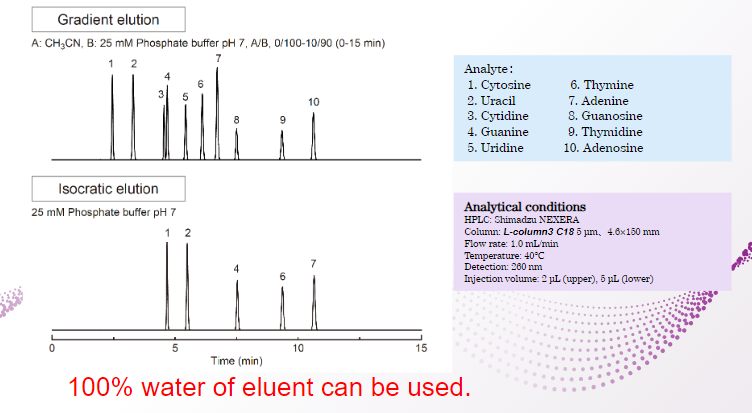
Normally, when C18column is used with a 100% aqueous eluent, the eluent that has penetrated into the pores is pushed out due to its hydrophobic properties when the flow is stopped.
Due to this phenomenon, the eluent cannot penetrate into the pores when the analysis is started again, resulting in inadequate retention.
However, L-column3 can perform stable analysis even when used with 100% aqueous eluent.
Therefore, it is possible to construct a 100% aqueous analysis method that can adequately retain even highly polar components.
Normally, when C18column is used with a 100% aqueous eluent, the eluent that has penetrated into the pores is pushed out due to its hydrophobic properties when the flow is stopped.
Due to this phenomenon, the eluent cannot penetrate into the pores when the analysis is started again, resulting in inadequate retention.
However, L-column3 can perform stable analysis even when used with 100% aqueous eluent.
Therefore, it is possible to construct a 100% aqueous analysis method that can adequately retain even highly polar components.
Comparison Between L-column Series and Other Columns
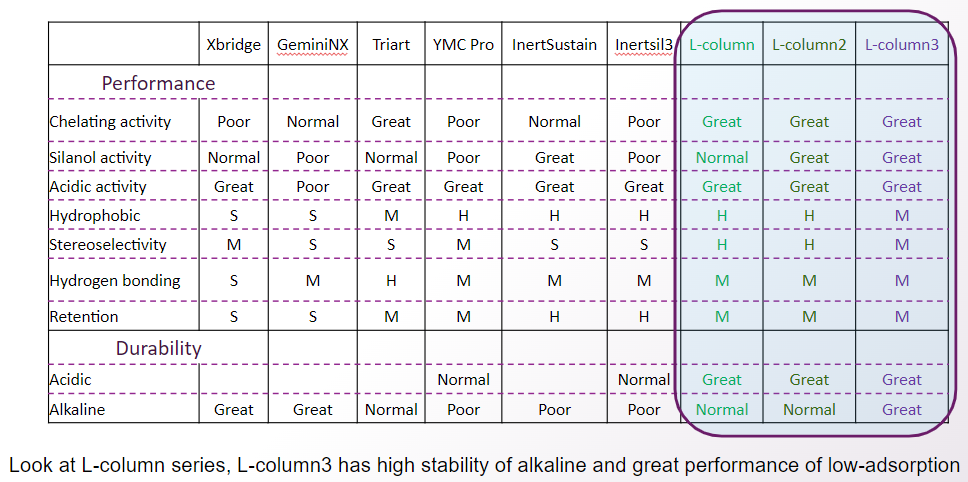
Focusing on the L-column series, L-column3 shows high stability against alkaline and excellent low adsorption.
On the other hand, hydrophobicity and stereoselectivity are slightly weaker.
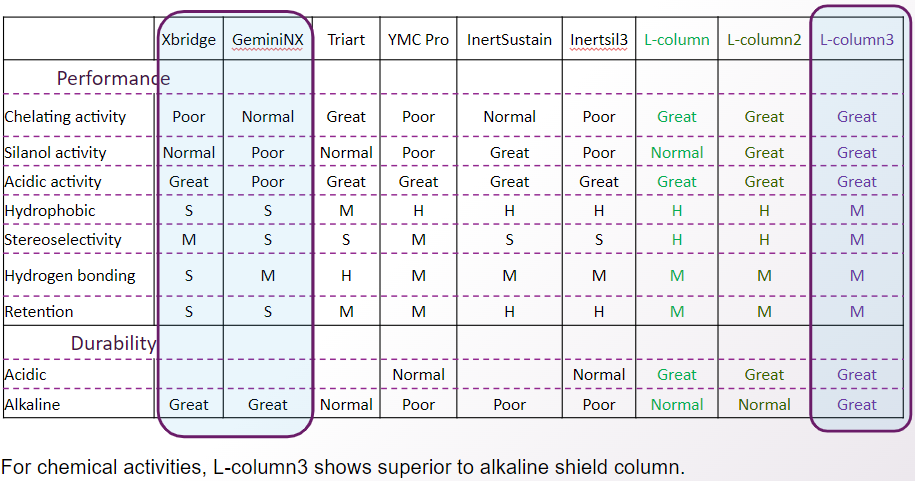
L-column3 has less metal coordination, and sharp peaks can be obtained even for acidic and basic substances.
This means that L-column 3 can be applied to a variety of analyses and is useful for analytical method development.
