20 Dec PROPER DEGASSING ENABLES
HIGH-PRECISION DISPENSING
OF CHILLED SOLUTIONS
Dispensing precision is critical to the performance of modern diagnostic kits, where repeatability and accuracy in the amount of reagent dispensed form the basis for reliable results. For manufacturing and production lines dispensing chilled solutions warming up presents a challenge as bubble formation caused by out-gassing threatens the precision. Deviation in the dispensed amount of solutions can affect the validity, economics, and possibly even the regulatory approval of a product.
In-line continuous degassing with Teflon® AF membranes effectively remove dissolved gasses from reagent solutions and avoids dispensing errors with greatly increased precision. Dispensing 1200 portions of water without degassing resulted in severe dispensing errors in 2% of the samples. An additional dispensing of a sampling of 800 portions of water with proper in-line degassing eliminated these dispensing errors.
Discussion
Current state-of-the-art diagnostic testing kits are designed to make the actual test procedure as straightforward and robust as possible, avoiding any end-user-induced mistakes. This frequently allows the patient to use the kit as a self-test leading to increased compliance and testing frequency resulting in significant savings to the healthcare system. A robust and easy-to-use test would be highly desirable for Covid-19 given the immense pressure on healthcare systems.
Releasing the demand on precision in sample dosing and switching from a sample-dependent response to the reagent, dependent response releases the burden of laboratory competence from the testing environment. On the other hand, it creates high demands on the precision of the dosing and dispensing of reagents building up the diagnostic kit. This is in most aspects very useful and effective but puts pressure on the manufacturing facility all the way from bulk containers to dispensing nozzles. Regardless of how carefully the fluidic path is designed, or which technology is used for the dispensing, dissolved gasses present a major challenge by readily forming microbubbles in the fluidic system. Bubble formation is typically induced by negative pressure transients in the pump or at the check valves. This tendency becomes even more pronounced when the aspiration speed is high as is very prevalent in high-throughput production situations.
Solubility of gasses in liquids generally decreases with increased temperature and reduced pressure as shown in Figure 1. From 4°C to 24°C, the solubility of air in water drops from 14.5 to 8.8 mg/mL and with that follows the potential to release 4.3 mL of gas bubbles per litre of water 1. The liberation of this gas can be held back by maintaining a positive pressure in the system but out-gassing will occur as soon as pressure is released. This typically happens during aspiration as a response to negative
pressure transients in pumps and check valves. This is also further catalysed by sharp edges and hydrophobic surfaces within the system.
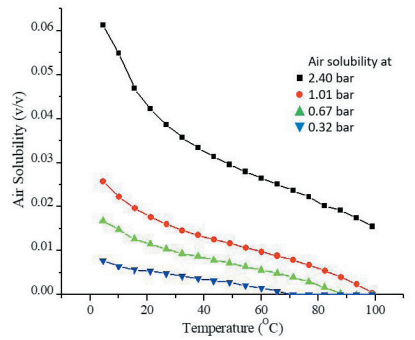
Figure 1: Solubility of air in water at different pressures as a function of temperature.
The solubility of air decreases by approximately 40% on warming up a refrigerated
solution to room temperature. A reduced pressure or negative pressure transients,
frequently produced by pumps, reduces the solubility even further resulting in
out-gassing and dispensing errors.
In-line Degassing
Removal of dissolved gasses long presented a significant challenge within the field of liquid chromatography (LC). Solutions used to address this challenge include vacuum degassing in-batch– with or without sonication – heating to reflux, or sparging with helium. It was early discovered that batch degassing was unable to provide reproducible results due to the quick reabsorption of gasses to the liquid. Continuous sparging with helium is very effective, with very low levels of residual gasses; however, it is a cost-prohibitive solution. As helium is a limited resource with several other important applications this is not a sustainable solution.
In-line degassing using gas-permeable membranes was introduced nearly 25 years ago in
high-performance liquid chromatography (HPLC) In a modern in-line degasser, the liquid
flow is passed through a Teflon® AF membrane situated within a vacuum chamber with a well-controlled vacuum (Figure 2). The degasser preferably operates on the low-pressure side of the system, before any pumps or mixers. In this way, each component is properly degassed before any dissolved gasses can disturb the pump work cycle. Over the years, degassing technology has become so well-established that degassing units are routinely built into most LC systems, which many users may not even realise.
The use of in-line degassers has also spread outside the field of liquid chromatography and is widely implemented in a range of analytical systems. One example includes biosensors such as surface plasmon resonance (SPR) detectors, where microbubbles disturb the readout by increased noise. Today, effective in-line degassers are available for flow rates from less than 1μL to 100mL per minute, chemically compatible with all regularly used solvents. A controlled and stable vacuum of 50 or 80 mmHg is supplied by small and virtually silent vacuum pumps, with integrated vacuum sensors such as the ZHCR pumps from IDEX Health & Science3, as shown in Figure 2.
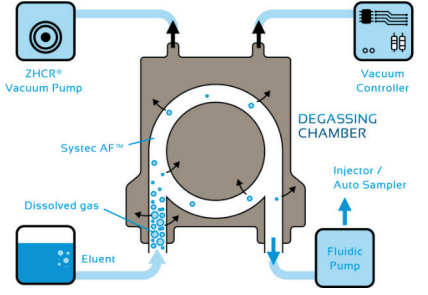
Figure 2: Schematic of the IDEX Health & Science Systec AF membrane of degassing
chambers (upper) and the in-line vacuum degasser (lower). The well-controlled
vacuum in the degassing chamber (brown) provides the driving force for the dissolved
gas to pass the degassing membrane (right).
Results
To demonstrate the power of proper degassing for increased precision and reproducibility during water dispensing, a validation experiment was performed with and without an in-line degasser in an otherwise identical system. Portions of 50 μL water saturated with air at room temperature were dispensed using a 500 μL syringe pump and the output was measured by gravity. The refill flow rate was set to 25 mL per minute, giving a syringe filling time of 1.2 seconds. In the degassing experiment, the degasser used a Teflon® AF tubular degassing membrane, with a length of 0.47 m and an internal volume of 480 μL, corresponding to one syringe volume. With the next aspiration portion of liquid standing still in the degasser tubing, the actual degassing occurred during the dispensing cycle of the preceding run.
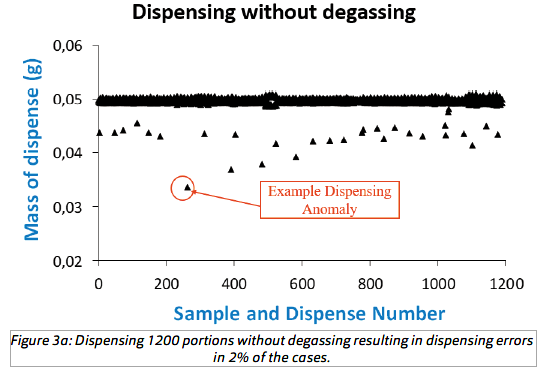
The result of dispensing 1200 portions of 50 μL water without degassing is shown in Figure 3a, (degasser off ). Dispensing anomalies (errors) were defined as giving less than 45 μL (90% of expected) occurred in 25 (2%) of the cases. Deviations of more than 2% by weight were observed in 96 (8%)
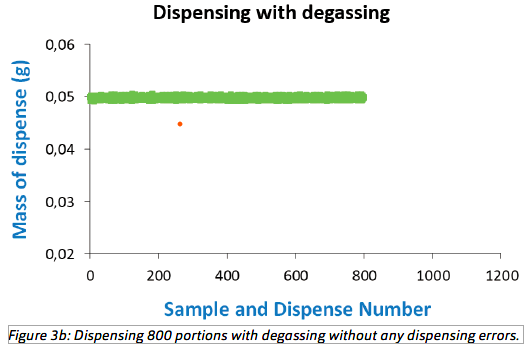
of the dispensing. In the next experiment, the procedure was repeated under identical conditions, but with an active degasser. The outcome from dispensing 800 portions of 50 μL water with active degassing
is shown in Figure 3b. With the degasser active, no dispensing anomaly was observed and no sample deviated more than 2% by weight from the target weight.
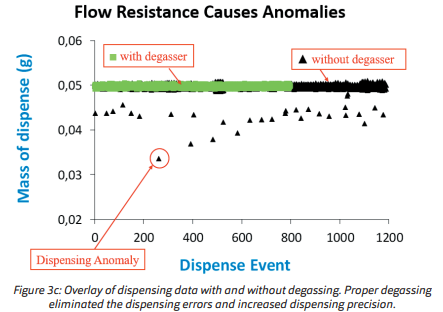

The difference between these experiments becomes even more apparent when an overlay is made
(Figure 3c). In each of these experiments, the temperature was kept constant during the full procedure.
Summary
We have demonstrated the power and importance of in-line degassing on dispensing outcomes without the additional driving force for out-gassing created by warming up the liquid. A chilled solution, saturated with air, gradually warms up during the process and should be expected to cause even more errors from bubble formation during dispensing if proper degassing is not applied. By proper degassing, not only can significant anomalies in data and dispensing be avoided, but the overall productivity of a manufacturing facility can increase. In this way, the root cause of dispensing anomalies is eliminated and precision is dramatically increased. The economic impact of this can outweigh the investment cost within weeks. Biotech AB and Biotech USA are the leading providers of in-line degassing solutions for flow rates from <1 μL/min to L/ min, and are available as standalone degassers (DEGASi®) as well as customised OEM solutions. These systems can handle a wide range of reagents, water-based solutions, and organic solvents4. We would like to express our gratitude to IDEX Health & Science for providing the data for the validation experiment.
REFERENCES
- The solubility of gasses in liquid is well described in Engineering
ToolBox, (2004). Air Solubility in Water. Available at: https://www.
engineeringtoolbox.com/air-solubility-water-d_639.html - The impact of dissolved gases on chromatography applications
- J.W. Dolan, LCGC Europe, Volume 27, Issue 7, p 307-352, 2014
- S.R. Bakalyar, M.P.T. Bradley and R. Honganen, “The Role
of Dissolved Gases in High-Performance Liquid Chromatography,” Journal of Chromatography A 158(1), 277–293 (1978) - J. Tokunaga, “Solubilities of Oxygen, Nitrogen and Carbon
Dioxide in Aqueous Alcohol Solutions,” J. Chem. Eng. Data 20(1),
41–46 (1975). - Flow-through vacuum degassing has been patented US6248157 B1
- DEGASi® is a registered trademark of Biotech AB, Onsala, Sweden.
www.biotechfluidics.com - Degassing chamber finder https://www.biotechfluidics.com/
degasi-finder/ - Vacuum pumps for degassing https://www.biotechfluidics.
com/products/degassing-debubbling/vacuum-pump-for-hplc- dispensing.

