212-204-0075
info@pyvot.tech
Separation of 6 Kinds of DPNH
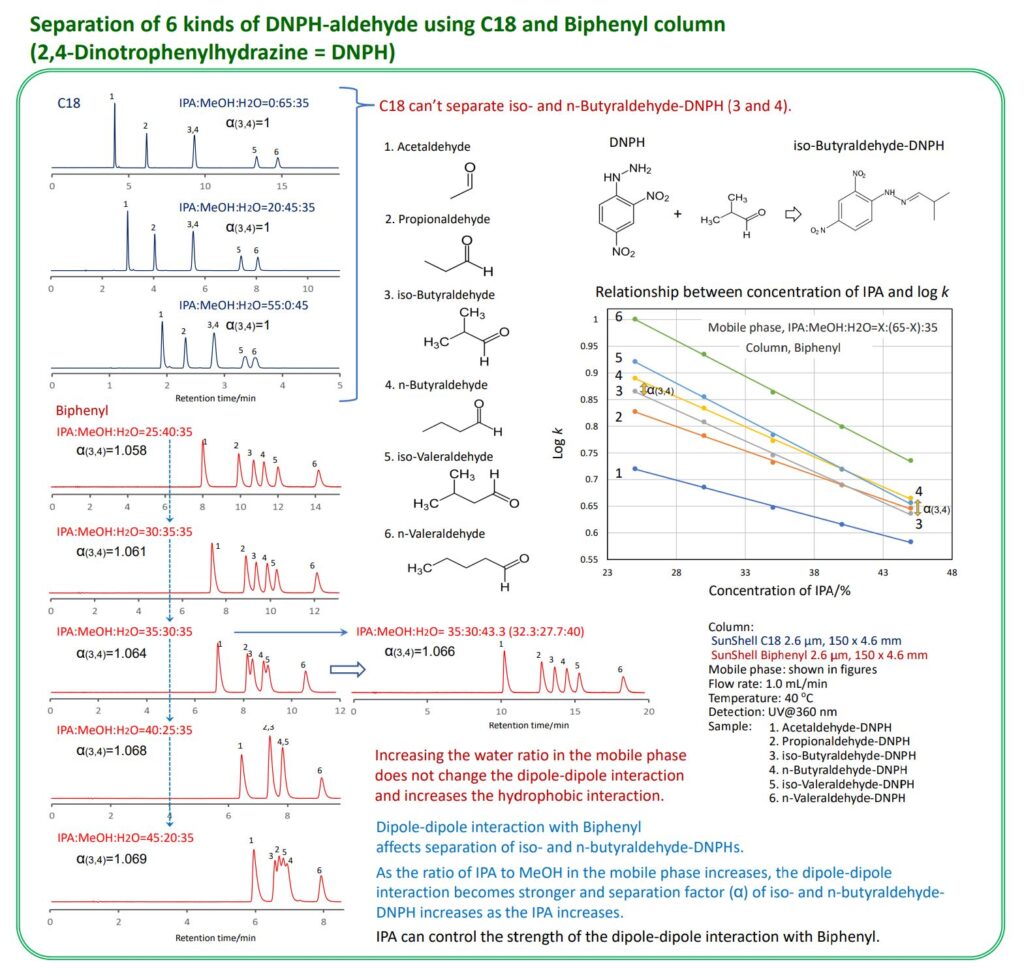
It is well-known that phenyl and biphenyl have dipole-dipole interactions that are absent in Alkyl phases such as C18 and C8. The biphenyl phase exhibits stronger dipole-dipole interaction than the phenyl phase, so the biphenyl phase was used for the effect of organic solvent on dipole-dipole interaction in this study. Methanol, 2-propanol, acetonitrile and tetrahydrofuran as an organic solvent in the mobile phase were compared to separate isomers of methylhippuric acid. It has been confirmed that acetonitrile and tetrahydrofuran counteract dipole-dipole interaction and 2-propanol enhances dipole-dipole interaction more than methanol. Additionally, retention time could be controlled by adding acetonitrile in the mobile phase. Although the C18 phase could not separate iso-butyraldehyde-DNPH and n-butyraldehyde-DHPH, the biphenyl phase could control the degree of separation of iso-butyraldehyde-DNPH and n-butyraldehyde-DNPH by changing the composition ration of methanol and IPA as organic solvents in the mobile phase.